Stuff • A coin A dropper • A bowl of water
DO

1. Place your coin on a flat, even surface.
____________________________________________________

2. Fill the dropper with water. Gently add one drop of water at a time on the coin.
____________________________________________________

3. Keep adding water to the coin until a small dome of water forms on it.
____________________________________________________

See
As you keep adding water to the coin, it continues to bulge and forms a dome on top of the coin, instead of flowing off it – though eventually it will.
____________________________________________________
Think about
Why did the water not spill over the edges?
This is because of a property of water called surface tension. The structure of water molecules is such that they stick tightly together. So, as more water is added on the coin, the water molecules pull each other inward and stick together strongly forming a dome.
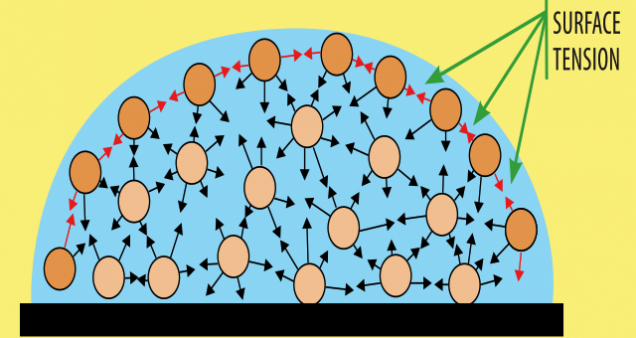
However, as you keep adding more water, the surface tension can no longer hold against the pull of gravity and the water eventually spills out.
Turn it into a game; see how many drops of water you can add till the dome breaks and challenge your friends to get more.
____________________________________________________
Let’s Find Out
How is surface tension useful?
Many waterproof tents are not exactly waterproof and yet the water still does not seep in. This is because the surface tension in the water droplets does not allow the water to flow through the pores of the tent material. But if you push against the water droplet, then it will go through the pores because the surface tension cannot hold against the force applied by your finger. You would have noticed this property of water on a rainy day—the water forms droplets against the window instead of spreading out.












COMMENT